Trip to South Africa
I recently travelled to South Africa to train with a collaborator to the Northern White Rhino Initiative, Dr. Morné de la Rey of EmbryoPlus. He has been working on a method to collect viable oocytes from living rhinoceros donors in a procedure called ‘ovum pick up’ (OPU).
Why you may ask?
With the oocytes extracted safely, they may be submitted to an in vitro system to mature, fertilize, and culture embryos in the incubator. If all of that goes successfully you end up with an embryo capable of transfer to a surrogate mother and a pregnancy the results in a baby. This process is performed routinely in human clinics and livestock fields.
The goal here is to develop a similar technique for rhinos so that more tools are available to save species.
Several hurdles exist in the process - as you can imagine. The in vitro culture conditions required for rhinos are rudimentary and need to be optimized, but in order to improve the culture conditions we need research and trials to determine the best media components, temperatures, and time frames – and their combinations.
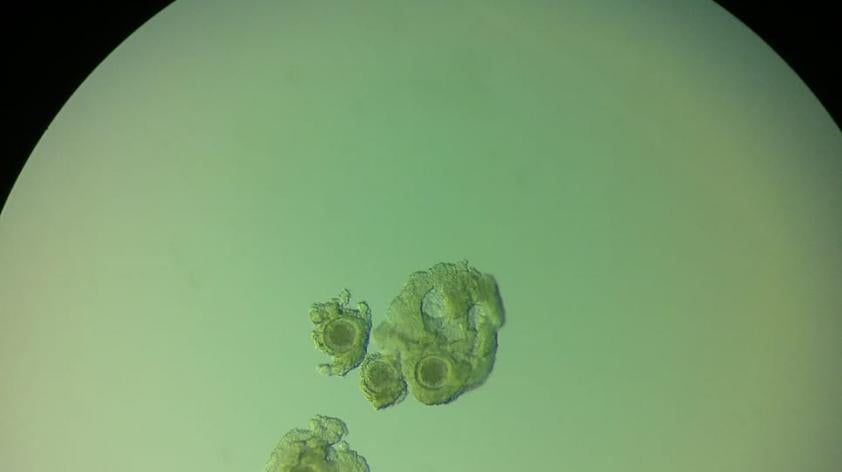
In order to do the research, we need the “raw materials”. In this case, oocytes. Sperm is also needed, but we can already successfully collect sperm from rhinos and store it frozen. Therefore, we need a reliable way to obtain oocytes as the material to use for in vitro culture trials. So, in order to get to production of rhino embryos in vitro, we need to start with obtaining oocytes from rhinos.
Oocytes may also be obtained from post mortem samples, but these are sporadically available and obviously requires the death of an animal – definitely not what we want. So, the avenue I will discuss here is OPU, but it is worth noting that it is not the only way to obtain oocytes.
OPU is utilized in several species on a regular basis already. Humans, as mentioned before, may undergo this technique as a fertility option. The process is also developed and utilized in cattle and horses, so the general idea is defined and outlined.
Briefly put, a needle is passed into a follicle on the ovary (follicles are structures that house the oocytes) and the follicle contents aspirated. Ideally, most of the follicles present are aspirated, recovering multiple oocytes per procedure. Those oocytes are then placed into a medium that promotes maturation whereby the oocyte becomes capable of fertilization.
The next step is fertilization of those that achieved ‘maturity’, then those that were successfully fertilized are placed into another culture medium to support further development. Not all of oocytes will achieve all of these steps, in fact it is common for 10 cultured oocytes to result in just 1 embryo in horses.
Knowing all of this, Dr. Morné has endeavored to develop a similar OPU technique in rhinos to provide the rhino oocytes needed for this critical research.
I had the opportunity in November to spend two weeks with him learning about all that he has accomplished. There has been much research and development into just the equipment design, but it seems he has begun to achieve some success. Just like learning any new skill, there is a learning curve associated with it.
Since I am familiar with the OPU procedure in horses, and he in cattle, we were able to hybridize the technique to build on the work he had already done. While I was there he recovered 17 oocytes from 8 total procedures on rhinos, averaging 4 oocytes per animal - a new record for him! The previous efficiency was 1 oocytes per animal.
It was a real pleasure to be a part of the development process and see it in action.













